Liquid chromatography (LC)¶
Chromatography is a technique used by life scientists to separate molecules based on a specific physical or chemical property.
Video
For more information on chromatography, view this video.
There are many types of chromatography, but this section focuses on LC as it is widely used in proteomics and metabolomics.
LC separates molecules based on a specific physical or chemical property by mixing a sample containing the molecules of interest (otherwise known as analytes) in a liquid solution.
Key components of LC¶
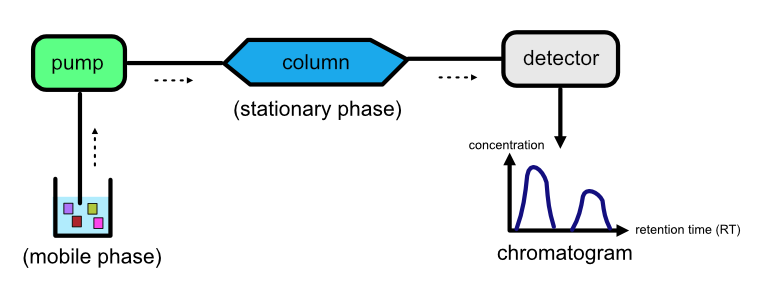
An LC setup is made up of the following components:
A liquid solution, known as the mobile phase, containing the analytes.
A pump which transports the liquid solution.
A stationary phase which is a solid, homogeneous substance.
A column that contains the stationary phase.
A detector that plots the time it takes for the analyte to escape the column (retention time) against the analyte’s concentration. This plot is called a chromatogram.
Refer to the image below for a diagrammatic representation of an LC setup.
How does LC work?¶
The liquid solution containing the analytes is pumped through a column that is attached to the stationary phase. Analytes are separated based on how strongly they interact with each phase. Some analytes will interact strongly with the mobile phase while others will be strongly attracted to the stationary phase, depending on their physical or chemical properties. The stronger an analyte’s attraction is to the mobile phase, the faster it will leave the column. The time it takes for an analyte to escape from the column is called the analyte’s retention time. As a result of their differing attractions to the mobile and stationary phases, different analytes will have different retention times, which is how separation occurs.
The retention times for each analyte are recorded by a detector. The most common detector used is the mass spectrometer, which we discuss later. However, other detection methods exist, such as:
Light absorption (photometric detector)
Fluorescence
Change in diffraction index
High performance liquid chromatography (HPLC)¶
HPLC is the most commonly used technique for separating proteins and metabolites. In HPLC, a high-pressured pump is used to transport a liquid (solvent) containing the molecules of interest through a thin capillary column. The stationary phase is ‘packed’ into the column.
Video
For more information on HPLC, view this video.
Several variations of HPLC exist such as:
Reversed-phase (RP) chromatography
Strong cation/anion exchange (SCX/SAX) chromatography
Affinity chromatography
Size exclusion chromatography
Special case of HPLC: Reversed-phase (RP) chromatography¶
RP chromatography is the most commony type of HPLC with biological samples. In reversed-phase liquid chromatography, the solid phase is modified to become hydrophobic, when it is originally hydrophilic, hence the term ‘reversed-phase’. The liquid phase is a mixture of water and an organic solvent. The separation of molecules happens based on the following behavior: hydrophilic analytes have a high affinity to the mobile phase and escape the column quickly while hydrophobic analytes have a high affinity towards the organic solvent and therefore, take a longer time to escape the column.
Video
For more information on RP chromatography, view this video.